Stereochemistry Drives the Macromolecular Conformation and Biological Activity of Glycopolymers
Authors:
Ishaq Muhammad Waqas, Parisa Farzeen, Lindsay R. Vaughn, Daniel J. Stone, Sanket A. Deshmukh, and Cassandra E. Callmann
Affiliation:
Muhammad Waqas Ishaq − Department of Chemistry, University of Texas at Austin, Austin, Texas 78712, United States
Parisa Farzeen − Department of Chemical Engineering, Virginia Tech, Blacksburg, Virginia 24061, United States
Lindsay R. Vaughn − Department of Chemistry, University of Texas at Austin, Austin, Texas 78712, United States
Daniel J. Stone − Department of Chemistry, University of Texas at Austin, Austin, Texas 78712, United States
Sanket A. Deshmukh − Department of Chemical Engineering, Virginia Tech, Blacksburg, Virginia 24061, United States
Description:
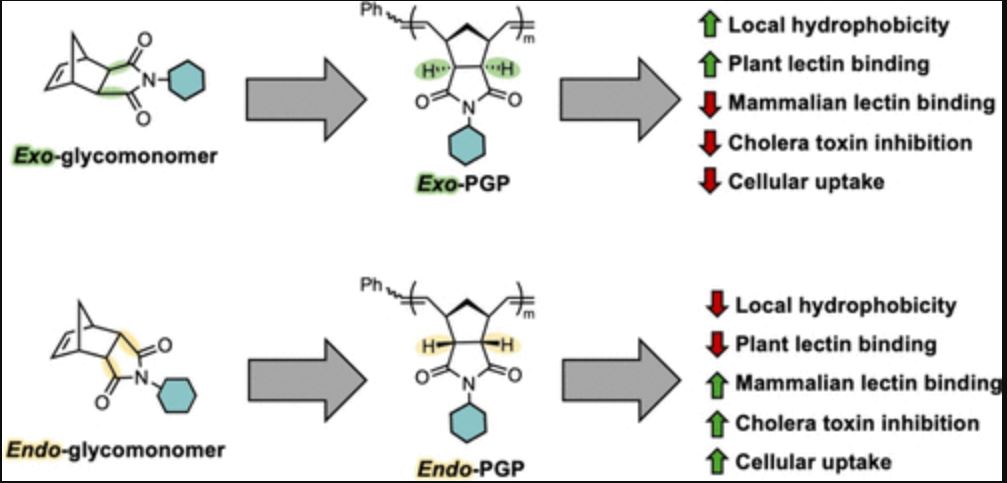
Chirality plays a fundamental role in biology, where stereochemical information governs how molecules fold, interact, and function. While the effects of stereochemistry are well-established for small molecules and natural biomacromolecules, less is known about how it shapes the properties of synthetic, biomimetic polymers. In this study, we explore how backbone and glycan stereochemistry influences conformation, physical interactions, and biological behavior in water-soluble glycopolymers. Using ring opening metathesis polymerization (ROMP), we synthesized precision glycopolymers (PGPs) from two diastereomeric norbornenyl moieties (endo and exo) and monosaccharides (glucose, galactose, and mannose). Despite having nearly identical molecular and macromolecular compositions, the resulting PGPs displayed major differences in their physical and biological properties. Glycopolymers with β-linkages showed distinct circular dichroism (CD) signals, and exo-derived backbones displayed more hydrophobic local environments, as confirmed by all-atom molecular dynamics simulations and dye interaction studies. These structural differences had clear functional consequences. exo-PGPs bound plant lectins more rapidly and with higher avidity, whereas endo-PGPs showed greater selectivity toward human galectin-3, stronger inhibition of cholera toxin, and enhanced uptake into 4T1 triple-negative breast cancer cells. Together, these findings provide the first demonstration of biological activity in endo-derived glycopolymers and establish backbone stereochemistry as a key design element that encodes macromolecular behavior in biologically relevant contexts.
Publications:
- Ishaq, Muhammad Waqas, Parisa Farzeen, Lindsay R. Vaughn, Daniel J. Stone, Sanket A. Deshmukh, and Cassandra E. Callmann; Stereochemistry Drives the Macromolecular Conformation and Biological Activity of Glycopolymers; ACS Central Science, 2025 — Available in VTechWorks
Tags:
CarbohydratesRelated Chronicles:
No related chronicles available
Files:
No related files available